BluePoint Labs Recalls Potassium Chloride Extended-Release Capsules
American Health Packaging on behalf of BluePoint Laboratories is recalling 21 batches of Potassium Chloride Extended-Release Capsules, USP (750 mg) 10 mEq K, to the consumer level citing failed dissol...
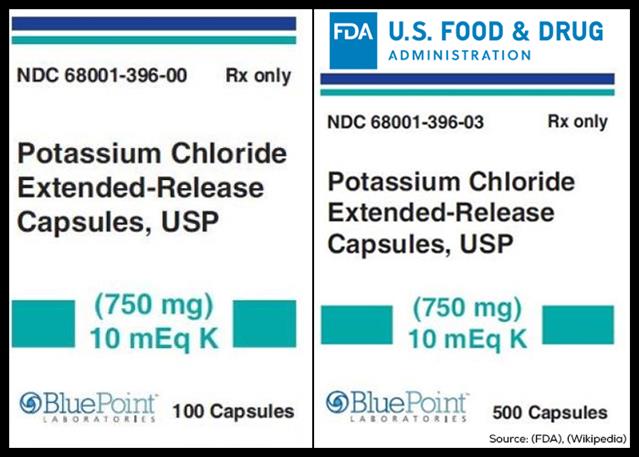
American Health Packaging on behalf of BluePoint Laboratories is recalling 21 batches of Potassium Chloride Extended-Release Capsules, USP (750 mg) 10 mEq K, to the consumer level citing failed dissolution, according to the U.S. Food and Drug Administration.
Potassium Chloride Extended-Release Capsules are used to treat patients with low potassium (hypokalemia).
They are packaged in bottles of 100-count (NDC 68001-396-00) and 500-count (NDC 68001-396-03) capsules, and were distributed nationwide to wholesale, distributor, and retail outlets.
Earlier this week, Mahwah, New Jersey-based Glenmark Pharmaceuticals Inc., USA had called back 114 batches of Potassium Chloride Extended-Release Capsules USP (750 mg) 10 mEq K citing failed dissolution.
The agency noted that the failed dissolution of potassium chloride extended release capsules may cause high potassium levels, also known as hyperkalemia, which can result in irregular heart beat that can lead to cardiac arrest.
To date, the firm has not received any reports of hyperkalemia or serious adverse events from spontaneous sources related to the recall.
Wholesalers, distributors, and retailers are urged to discontinue distribution of the recalled product lots immediately.
Consumers with the capsules are asked to consult with their physician or health care provider before they stop using the product.
For More Such Health News, visit rttnews.com
- Check out our free forex signals
- Follow the top economic events on FX Leaders economic calendar
- Trade better, discover more Forex Trading Strategies
- Open a FREE Trading Account